Why does it take so much time for developing medicine for viruses such as SARS, SARS-CoV-2, H1N1?
One of the main reason is because of time consumed in clinical trails in animal and then on humans. Data from these trial leads to development of vaccine for the virus.
What is Clinical Research or Trials?
Clinical trials are conducted to collect data regarding the safety and efficacy of new drug and device development. There are several steps and stages of approval in the clinical trials process before a drug or device can be sold in the consumer market.
The trial sponsor, often the pharmaceutical company that develops the therapy or medication, prepares the protocol for the clinical trial. A protocol is a plan that explains how the trial will work, what will be done during the trial, and why. Each medical center that conducts the trial uses the same protocol.
Key information in a protocol includes:
1. How many patients will participate
2. Who is eligible
3. What tests patients will get and how often
4. What type of data will be collected
5. Detailed information about the treatment plan, if appropriate
Importance of Randomization
Clinical researchers work to ensure that they avoid bias in clinical trials. Bias refers to human choices or other factors (unrelated to the protocol) that might affect the trial’s results.
Randomization helps ensure that researchers don’t introduce bias into the trial. In many clinical trials that test the effectiveness of a medication, half of the participants receive the medication in question. The other half receive a placebo, which contains no medication. Randomization involves assigning patients to these comparison groups by chance, rather than choice.
The Four Phases of Clinical Research
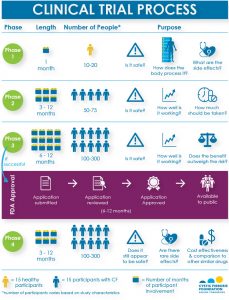
Who Can Participate?
All clinical trials have guidelines about who can join. Some enroll healthy people. Others enroll only people with certain conditions, such as CF.
COVID-19 Clinical Trail- How far are we?
COVID-19 is a disease caused by SARS-CoV-2 virus. It has infected 536,906 people all over the world and has claimed 24,124 lives till 27/03/2020.
About 35 companies and academic institutions are racing to create vaccine, at least four of which already have candidates they have been testing in animals. The first of these – produced by Boston-based biotech firm Moderna – will enter human trials imminently.
Norway-based CEPI, the Coalition for Epidemic Preparedness Innovations has already provided funding to several organizations and institutions working on vaccines, including
1. Inovio Pharmaceuticals: Its DNA-based vaccine has begun pre-clinical trials, meaning the company is ready to test its candidate on humans.
2. GlaxoSmithKline: Developing a “molecular clamp” vaccine, which would contain the protein that enables COVID-19 to enter human cells. This method was worked up by the University of Queensland in Australia.
3. Moderna: The US company has already begun testing an RNA-based treatment.
Still with all these effort ,time to get a COVID-19 vaccine to market is likely to be at least 18 months away. Most of the vaccines we rely on today took between five and 15 years to perfect. So even if we go very fast using present technology it will take around 1.5 years to make vaccine so that it will not produce harmful side effect on humans.
For the time being, the best way to ensure you reduce your risk of infection is to follow the World Health Organization’s advice on hand washing and social distancing.
Stay Home, Stay Safe.